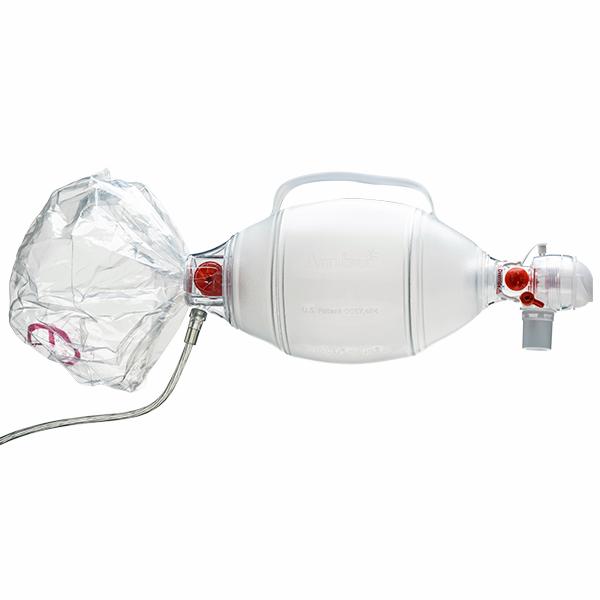
Ambu® SPUR® II - Disposable Resuscitator
Ambu SPUR II provides users with exceptional tactile and visual feedback during resuscitation. The bag is highly responsive, featuring minimal mechanical resistance. Moreover, the characteristic design provides the optimum in respect to stroke volume and recoil.
Key benefits
General information
Ambu SPUR II is the only single use resuscitator that is made from a SEBS polymer instead of PVC. This classifies Ambu SPUR II as environmentally safe and fully disposable, thus eliminating all risk of cross contamination. It is part of a complete family of resuscitators, including infant, pediatric, and adult sizes.
All Ambu SPUR II resuscitators come in individual, resealable carrying bags, complete with one or more masks and any special accessories – color-coded for fast size identification.
Always read the instructions for use (IFU).
Suitable applications
Manual ventilation and resuscitation.
Spare parts
We offer 1 Spare parts for this productDownloads
Brochures
(2.65 MB - pdf)
Datasheets
(90.86 KB - pdf)
Instructions for use
(1.39 MB - pdf)
Supplementary Information
(2.31 MB - pdf)
TGA Certificates
(998.18 KB - pdf)
(142.07 KB - pdf)
(998.27 KB - pdf)
April 2019